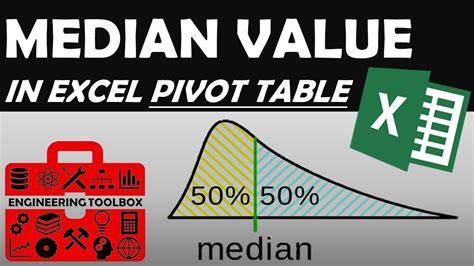
What You Need to Know About Fahrenheit's Freezing Point

The freezing point of water, a fundamental concept in science and everyday life, is often associated with the temperature scale invented by Daniel Gabriel Fahrenheit. While Celsius and Kelvin are the more commonly used scales today, understanding Fahrenheit’s freezing point is essential for a comprehensive grasp of temperature measurement. This article delves into the intricacies of this temperature marker, exploring its historical context, scientific significance, and practical applications.
Historical Evolution of Fahrenheit’s Scale
Daniel Gabriel Fahrenheit, a German-Dutch physicist, developed his temperature scale in the early 18th century. Fahrenheit’s scale was one of the earliest practical temperature measurement systems and was based on the freezing and boiling points of water. He chose these points as fixed reference points for his scale, a practice that was innovative at the time.
Fahrenheit’s scale was initially designed to facilitate accurate meteorological observations and to improve upon the existing temperature scales of his time, which lacked uniformity and precision. He set the freezing point of water at 32 degrees Fahrenheit and the boiling point at 212 degrees Fahrenheit. This scale was widely adopted in Europe and the Americas for over two centuries, becoming a familiar unit of measurement in daily life.
Scientific Significance and Practical Applications

The freezing point of water at 32 degrees Fahrenheit is a critical reference point in various scientific and industrial processes. Here’s a closer look at its significance:
Biology and Medicine: Understanding the freezing point of water is crucial in biology and medicine. Many biological processes are sensitive to temperature, and the freezing point of water is a reference point for preserving biological samples and maintaining the stability of biological systems.
Environmental Science: In environmental science, the freezing point of water plays a vital role in understanding weather patterns, climate change, and ecological processes. It helps in studying the formation of ice, snow, and glaciers, and the impact of freezing temperatures on ecosystems.
Food Science and Preservation: The freezing point is a critical factor in food preservation. Food safety and quality are closely tied to temperature control, and understanding the freezing point of water is essential for maintaining the freshness and safety of perishable goods.
Chemical Engineering: Chemical processes often involve precise temperature control. The freezing point of water serves as a reference for temperature measurements and the design of chemical processes. It is especially important in industries like pharmaceuticals, where temperature control is critical to product quality.
Transportation and Infrastructure: In regions with harsh winters, understanding the freezing point of water is crucial for maintaining transportation systems and infrastructure. For example, the freezing point of water impacts the design of bridges and roads, as well as the management of ice and snow on these structures.
Practical Guidance and Safety Considerations
While the freezing point of water at 32 degrees Fahrenheit is a fixed reference point, it’s essential to recognize that the actual behavior of water can vary based on external factors:
Pressure: The freezing point of water is influenced by pressure. At high altitudes, where air pressure is lower, water may freeze at temperatures below 32 degrees Fahrenheit. This is why ice skating is possible in locations like Colorado, despite its relatively high altitude.
Salinity: The presence of dissolved salts in water, known as salinity, can also affect the freezing point. Seawater, for instance, has a lower freezing point than freshwater due to the salts it contains. This is why oceans and seas don’t freeze solid, even in very cold climates.
Supercooled Water: Water can sometimes be “supercooled” below its freezing point without turning into ice. This phenomenon occurs when water is free of impurities and is kept in a very clean, undisturbed state. Supercooled water can be a safety hazard, as it can rapidly turn into ice when disturbed, causing sudden freezing and potential hazards.
Comparative Analysis: Celsius and Kelvin Scales
While Fahrenheit’s scale has its own merits, the Celsius and Kelvin scales are more widely used in scientific and international contexts. Here’s a comparison:
| Scale | Freezing Point of Water | Boiling Point of Water |
|---|---|---|
| Fahrenheit | 32°F | 212°F |
| Celsius | 0°C | 100°C |
| Kelvin | 273.15K | 373.15K |

The Celsius scale, developed by Anders Celsius, is simpler and more intuitive for most people, as it uses a base-10 system. The Kelvin scale, on the other hand, is used in scientific contexts because it starts at absolute zero, making it ideal for measuring extremely low temperatures.
Expert Perspective: Future Trends and Innovations
As we move towards a more sustainable and efficient future, temperature measurement and control will play an increasingly important role. Experts predict that advancements in temperature measurement technology will lead to more precise and reliable systems, especially in critical industries like medicine, energy, and environmental science.
One emerging trend is the development of smart temperature sensors that can provide real-time data and alerts. These sensors can be integrated into various systems, from home thermostats to industrial processes, enabling more efficient and responsive temperature control.
Additionally, there is growing interest in developing temperature-responsive materials that can change their properties based on temperature fluctuations. These materials have the potential to revolutionize fields like energy storage, where efficient heat management is critical.
In conclusion, understanding the freezing point of water at 32 degrees Fahrenheit is a cornerstone of temperature measurement. While Fahrenheit’s scale may not be as widely used today, its historical significance and practical applications continue to make it an essential concept in science and everyday life.
Mastering the fundamentals of temperature measurement, such as the freezing point of water, empowers us to navigate the complexities of the natural world and harness its potential for a sustainable future.