Unveiling the Mystery of Endothermic Reactions
Diving into the world of chemical reactions, we often encounter the intriguing phenomenon known as endothermic reactions. These reactions, which absorb heat from their surroundings, present a unique contrast to the more common exothermic reactions that release heat. In this article, we will embark on a journey to unravel the secrets of endothermic reactions, exploring their mechanisms, applications, and the intriguing role they play in various scientific disciplines.
Understanding the Fundamentals: What are Endothermic Reactions?

At its core, an endothermic reaction is a chemical process that requires an input of energy, specifically in the form of heat, to proceed. This energy input is necessary to overcome the activation energy barrier, enabling the reaction to occur. In essence, the reactants absorb heat from their environment, leading to a decrease in the temperature of the surroundings. This unique characteristic sets endothermic reactions apart and makes them an essential concept in the study of thermodynamics.
The Thermodynamic Perspective
From a thermodynamic standpoint, endothermic reactions are associated with a positive enthalpy change (ΔH > 0). This indicates that the system absorbs heat energy from its surroundings during the reaction. The absorbed heat is utilized to break the chemical bonds in the reactants, facilitating the formation of new bonds and the creation of products. The concept of endothermicity is deeply rooted in the principles of energy conservation and the first law of thermodynamics.
Examples in Action
To grasp the concept more concretely, let’s consider a few examples of endothermic reactions:
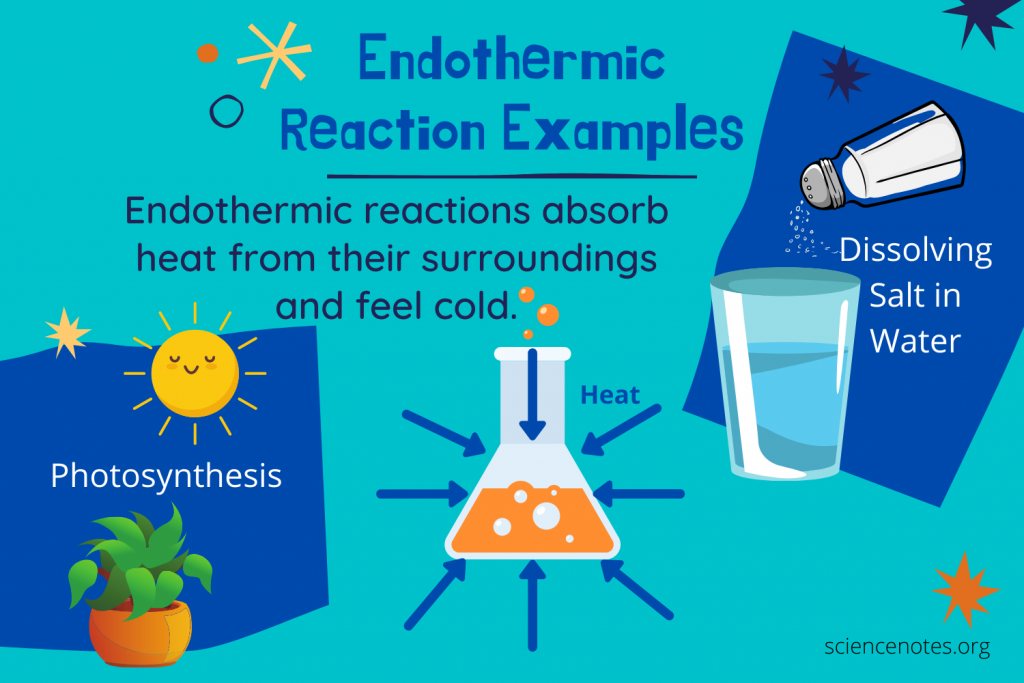
Dissolving Ammonium Nitrate in Water: When ammonium nitrate (NH4NO3) dissolves in water, it absorbs heat from the surroundings, causing the solution to feel cold. This reaction is often utilized in cold packs to provide relief from muscle soreness or injuries.
Photosynthesis in Plants: One of the most remarkable endothermic processes occurs in nature—photosynthesis. Plants absorb sunlight (energy) to convert carbon dioxide and water into glucose and oxygen. This reaction is vital for sustaining life on Earth.
Melting Ice: The process of melting ice is another classic example. When ice cubes are added to a drink, they absorb heat from the liquid, leading to a decrease in the drink’s temperature. This is why ice cubes are often used to keep beverages cool.
Unraveling the Mechanism: How Endothermic Reactions Work
Delving deeper into the intricacies of endothermic reactions, we explore the mechanisms that govern these processes. Understanding the underlying principles is crucial for both theoretical comprehension and practical applications.
Activation Energy and Reaction Barriers
The initiation of any chemical reaction, including endothermic reactions, requires overcoming an activation energy barrier. This barrier represents the minimum energy required for the reactants to transform into products. In endothermic reactions, the activation energy is higher than the energy available in the system, leading to the need for an external energy input.
Energy Diagrams: Visualizing the Process
Energy diagrams provide a valuable tool for visualizing the energy changes during a chemical reaction. In the case of endothermic reactions, the energy diagram exhibits a rise in energy from the reactants to the transition state, followed by a decrease in energy as the products are formed. This rise in energy corresponds to the energy input required to overcome the activation barrier.
Factors Influencing Endothermicity
Several factors can influence the endothermic nature of a reaction:
Bond Strength: Reactions involving the breaking of strong chemical bonds are more likely to be endothermic. The energy required to break these bonds is substantial and often exceeds the energy available in the system.
Reactant Stability: The stability of the reactants plays a crucial role. Less stable reactants tend to have lower activation energies, making them more prone to endothermic reactions.
Reaction Conditions: External factors such as temperature, pressure, and the presence of catalysts can impact the endothermicity of a reaction. Changes in these conditions can influence the energy requirements and the overall thermodynamics of the process.
Real-World Applications: Where Endothermic Reactions Shine
Beyond the theoretical understanding, endothermic reactions find practical applications in various fields, showcasing their versatility and importance.
The Chemistry of Endothermic Reactions
Inorganic Chemistry: Endothermic reactions are fundamental in the synthesis of many inorganic compounds. For instance, the production of ammonia (NH3) through the Haber-Bosch process is an endothermic reaction that plays a vital role in the fertilizer industry.
Organic Chemistry: Organic reactions often involve endothermic steps. The Diels-Alder reaction, a classic example in organic synthesis, is endothermic and utilized in the preparation of complex molecules.
Environmental Science: Endothermic processes are integral to understanding and addressing environmental challenges. For example, the endothermic nature of certain reactions is harnessed in the development of alternative energy sources like hydrogen fuel cells.
Industrial Applications
Refrigeration and Air Conditioning: Endothermic reactions are at the heart of refrigeration systems. The absorption of heat from the surroundings by a refrigerant is an essential step in maintaining cool temperatures in refrigerators and air conditioners.
Chemical Manufacturing: In the chemical industry, endothermic reactions are employed in processes such as the synthesis of pharmaceuticals, polymers, and specialty chemicals. These reactions often require precise control of energy input to achieve desired outcomes.
Food Processing: The endothermic nature of certain reactions is leveraged in food preservation. For instance, the freezing of foods is an endothermic process that slows down microbial activity, extending the shelf life of perishable items.
Case Study: Endothermic Reactions in Environmental Remediation
To illustrate the practical impact of endothermic reactions, let’s explore a real-world case study: the use of endothermic processes in environmental remediation.
Cleaning Up Contaminated Sites
In the field of environmental science, endothermic reactions are employed to address soil and groundwater contamination. One notable example is the use of in situ thermal desorption (ISTD) technology.
How ISTD Works
ISTD involves heating the contaminated soil or groundwater to high temperatures, causing the contaminants to vaporize. This endothermic process effectively removes pollutants, such as volatile organic compounds (VOCs) and semi-volatile organic compounds (SVOCs), from the environment. The absorbed heat energy breaks down the chemical bonds of the contaminants, allowing for their safe removal and disposal.
Benefits and Considerations
The advantages of ISTD include:
Efficient Remediation: ISTD can rapidly reduce the concentration of contaminants, making it an effective solution for time-sensitive situations.
Minimal Site Disruption: Unlike traditional excavation methods, ISTD can be performed in situ, minimizing the disturbance to the environment and surrounding infrastructure.
However, ISTD also presents certain challenges:
Energy Requirements: The endothermic nature of the process demands a significant energy input, which can be costly and resource-intensive.
Site-Specific Considerations: The success of ISTD depends on various factors, including the nature of the contaminants, soil characteristics, and the presence of other chemicals.
Future Trends: Exploring Emerging Endothermic Technologies
As scientific research continues to advance, new technologies and applications for endothermic reactions are emerging. These developments hold the potential to revolutionize various industries and address global challenges.
Solar-Driven Endothermic Reactions
One exciting area of exploration is the use of solar energy to power endothermic reactions. Researchers are investigating the potential of solar-thermal systems to provide the necessary energy input for endothermic processes. This approach not only reduces the reliance on traditional energy sources but also aligns with sustainable and renewable energy goals.
Advanced Materials and Catalysts
The development of advanced materials and catalysts is another area of focus. Scientists are working on designing materials that can enhance the efficiency of endothermic reactions, reducing the energy requirements and improving overall performance. These materials could find applications in various fields, from energy storage to chemical manufacturing.
Conclusion: Unlocking the Potential of Endothermic Reactions
In conclusion, endothermic reactions are not merely a theoretical concept but a powerful tool with wide-ranging applications. From the fundamental principles of thermodynamics to real-world industrial processes, endothermicity plays a pivotal role in shaping our understanding of chemical reactions. As we continue to explore and innovate, the potential for harnessing endothermic reactions to solve complex problems and drive sustainable solutions becomes increasingly evident.
Key Takeaways:
- Endothermic reactions absorb heat energy from their surroundings, leading to a decrease in temperature.
- These reactions are characterized by a positive enthalpy change (ΔH > 0) and require an external energy input to overcome activation barriers.
- Endothermic reactions find applications in diverse fields, including chemistry, environmental science, and industrial processes.
- Emerging technologies, such as solar-driven endothermic reactions and advanced materials, hold promise for further advancements in this field.
- Understanding endothermic reactions is crucial for both theoretical comprehension and practical problem-solving.
FAQ:
What is the difference between endothermic and exothermic reactions?
+Endothermic reactions absorb heat energy from their surroundings, resulting in a decrease in temperature. In contrast, exothermic reactions release heat energy, causing an increase in the temperature of the surroundings.
Can endothermic reactions be used to generate heat?
+While endothermic reactions absorb heat, they do not generate heat in the traditional sense. However, by harnessing the absorbed heat energy and converting it into a usable form, such as electricity or mechanical work, heat can be indirectly produced.
Are all endothermic reactions slow and inefficient?
+No, not all endothermic reactions are slow or inefficient. The speed and efficiency of a reaction depend on various factors, including the activation energy, temperature, and the presence of catalysts. Some endothermic reactions can be quite rapid and efficient under the right conditions.
What are some everyday examples of endothermic reactions?
+Everyday examples include the dissolution of ammonium nitrate in water, the melting of ice, and the process of photosynthesis in plants. These reactions demonstrate the endothermic nature of certain processes we encounter in our daily lives.
How do endothermic reactions impact the environment?
+Endothermic reactions can have both positive and negative impacts on the environment. Some endothermic processes, like photosynthesis, are vital for sustaining life and maintaining ecological balance. However, certain industrial endothermic reactions can contribute to energy consumption and carbon emissions if not properly managed.
By delving into the world of endothermic reactions, we uncover a fascinating aspect of chemistry and its practical applications. As research progresses, the potential for innovative solutions and sustainable practices powered by endothermicity becomes increasingly promising.