Unveiling Enzyme Complex's Substrate Secrets
The intricate world of enzyme-substrate interactions has long fascinated biochemists and molecular biologists, offering a glimpse into the intricate machinery of life itself. At the heart of this biological ballet, enzyme complexes play the role of conductors, orchestrating the transformation of substrates into products with remarkable precision and efficiency. But how do these complexes unlock the secrets of their substrates? Let’s embark on a journey to uncover the mysteries that lie within.
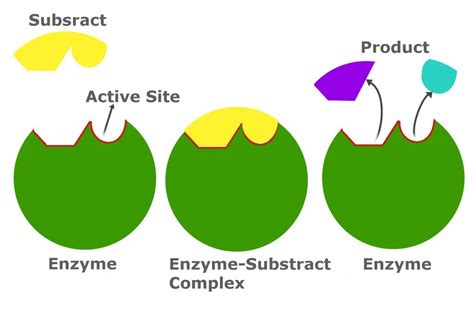
Enzyme-substrate interactions are the cornerstone of biochemical reactions, facilitating the transformation of molecules into new forms. These interactions are highly specific, akin to a lock and key, where the enzyme serves as the key and the substrate as the lock.
The Substrate’s Journey: From Arrival to Transformation
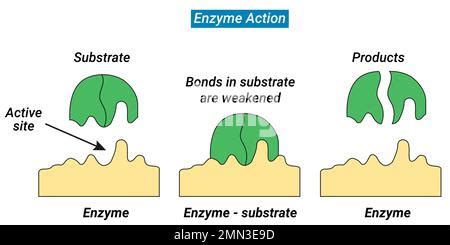
Imagine a bustling molecular city, where substrates, akin to travelers, arrive at the enzyme complex’s gate, seeking entry and transformation. But how does this intricate process unfold? Let’s delve into the steps that define this remarkable journey.
Step 1: Recognition and Binding
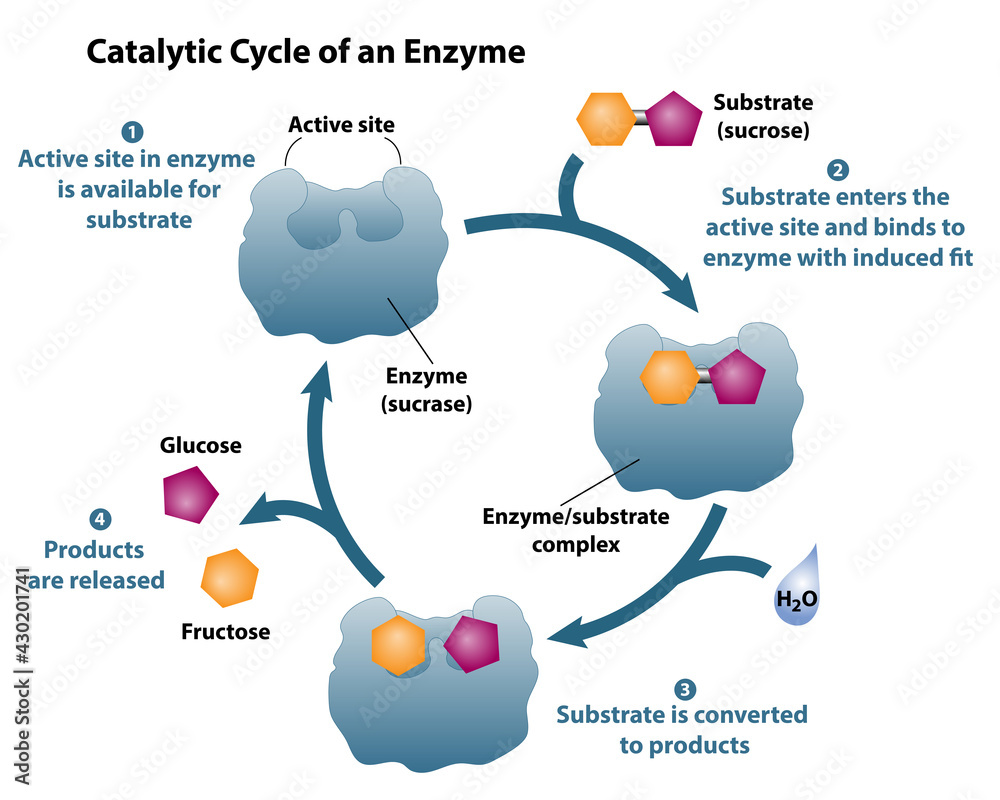
In the bustling molecular city, the enzyme complex stands as a vigilant gatekeeper, recognizing its substrate through a highly specific binding process. This initial recognition is akin to a secret handshake, where the enzyme and substrate must fit together perfectly for the interaction to proceed.
Enzyme-substrate recognition is a highly specific process, driven by the unique molecular structure of both partners. This specificity ensures that only the intended substrate can bind to the enzyme, preventing unwanted reactions.
Step 2: Induced Fit and Conformational Changes
Upon successful binding, the enzyme complex undergoes a remarkable transformation, akin to a dancer adapting to their partner’s movements. This process, known as induced fit, sees the enzyme complex adjusting its shape to accommodate the substrate, creating a perfectly tailored environment for the upcoming reaction.
Pros of Induced Fit
- Ensures a precise fit between enzyme and substrate, enhancing specificity.
- Allows for efficient catalysis by bringing reactive groups into close proximity.
Cons of Induced Fit
- Requires additional energy for conformational changes, increasing the reaction's overall energy cost.
- Might lead to reduced flexibility in accommodating diverse substrates.
Step 3: Catalysis and Transformation
With the enzyme complex and substrate now perfectly aligned, the stage is set for the catalytic event. The enzyme complex, acting as a skilled choreographer, guides the substrate through a series of precise movements, facilitating the transformation into the desired product. This catalytic process is remarkably efficient, often accelerating reaction rates by millions of times compared to uncatalyzed reactions.
The catalytic power of enzyme complexes lies in their ability to lower the activation energy barrier, making it easier for substrates to undergo the necessary molecular rearrangements for transformation.
Step 4: Product Release and Departure
As the catalytic process reaches its conclusion, the enzyme complex prepares for the departure of its product. In a carefully choreographed movement, the product is released, freeing up the enzyme complex to welcome a new substrate and begin the cycle anew. This continuous process ensures the efficient conversion of substrates into products, driving the intricate dance of life forward.
Unlocking the Secrets: Key Factors and Mechanisms
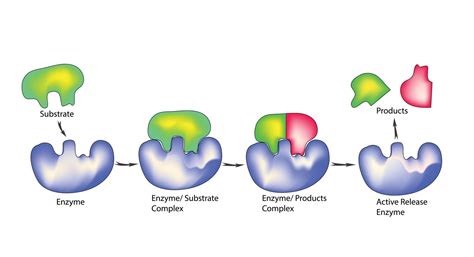
The successful interaction between enzyme complexes and their substrates is a complex dance, influenced by a myriad of factors and mechanisms. Let’s explore some of the key elements that contribute to this intricate choreography.
Enzyme-Substrate Affinity
Affinity, a measure of the strength of attraction between enzyme and substrate, plays a pivotal role in the enzyme-substrate interaction. High affinity, characterized by a low dissociation constant (Kd), indicates a strong attraction, ensuring efficient binding and reaction progression. Conversely, low affinity, with a higher Kd, may lead to reduced binding efficiency and potentially slower reaction rates.
How does enzyme-substrate affinity impact reaction rates?
+Enzyme-substrate affinity is a critical factor influencing reaction rates. Higher affinity, characterized by a low dissociation constant (Kd), ensures efficient binding, leading to faster reaction rates. Conversely, lower affinity may result in slower reactions due to reduced binding efficiency.
Substrate Specificity
Substrate specificity, a measure of an enzyme’s ability to recognize and act upon a specific substrate, is a key factor in ensuring the precision of biochemical reactions. Enzyme complexes are highly selective, often recognizing only a single substrate or a small group of structurally similar substrates. This specificity prevents unwanted reactions and ensures that the enzyme’s catalytic power is directed towards the intended transformation.
Case Study: The Specificity of Trypsin
Trypsin, a proteolytic enzyme, is a prime example of substrate specificity. It specifically recognizes and cleaves peptide bonds on the carboxyl side of arginine and lysine residues. This high specificity ensures that trypsin efficiently breaks down proteins into smaller peptides, playing a crucial role in digestion.
Catalytic Power and Efficiency
The catalytic power of enzyme complexes is a marvel of nature, allowing for the acceleration of biochemical reactions by millions of times. This extraordinary efficiency is achieved through a combination of factors, including induced fit, which brings reactive groups into close proximity, and the unique chemical properties of the enzyme’s active site, which lower the activation energy barrier.
Enzyme complexes are nature's ultimate catalysts, possessing the remarkable ability to accelerate biochemical reactions by lowering activation energy barriers and guiding substrates through precise molecular transformations.
Exploring the Enzyme Complex’s Toolbox
Enzyme complexes are molecular Swiss Army knives, equipped with a diverse set of tools to facilitate their catalytic roles. Let’s explore some of the key components that contribute to their remarkable efficiency and specificity.
Active Site: The Heart of Catalysis
The active site of an enzyme complex is its most precious asset, a carefully crafted molecular environment designed to facilitate the transformation of substrates into products. This site is characterized by a unique combination of amino acid residues, each contributing specific chemical and physical properties that lower the activation energy barrier and guide the substrate through its catalytic journey.
Advantages of Active Site Design
- Provides a highly specific environment for substrate binding and transformation.
- Enables precise control over reaction conditions, ensuring efficiency and specificity.
Challenges of Active Site Design
- May limit the enzyme's ability to accommodate diverse substrates, reducing flexibility.
- Requires precise molecular engineering, making it challenging to design artificial enzymes.
Coenzymes and Cofactors: Enhancing Catalytic Power
Enzyme complexes often rely on coenzymes and cofactors to enhance their catalytic power. These small molecules, acting as helpers, participate in the catalytic process, often by providing essential chemical groups or facilitating critical molecular rearrangements. Examples include NADH, which acts as a hydrogen carrier in numerous oxidation-reduction reactions, and ATP, the energy currency of the cell.
| Coenzyme/Cofactor | Function |
|---|---|
| NADH | Acts as a hydrogen carrier in oxidation-reduction reactions. |
| ATP | Provides energy for numerous biochemical reactions. |
| Vitamin B12 | Facilitates critical reactions in DNA synthesis and methylation. |
| Metal Ions (e.g., Zn2+) | Provide structural stability and catalytic activity to enzymes. |

Allosteric Regulation: Fine-Tuning Enzyme Activity
Allosteric regulation is a sophisticated mechanism that allows enzyme complexes to fine-tune their activity in response to changing cellular conditions. This process involves the binding of a regulatory molecule, often a small effector molecule or another protein, to a site on the enzyme complex distinct from the active site. This binding triggers conformational changes that alter the enzyme’s activity, either enhancing or inhibiting its catalytic power.
Steps in Allosteric Regulation
- Binding of regulatory molecule to allosteric site.
- Induced conformational changes in the enzyme complex.
- Alteration of enzyme activity, either activation or inhibition.
- Impact on biochemical reactions, influencing cellular processes.
Conclusion: Unlocking the Secrets, Unveiling the Wonders
The intricate dance between enzyme complexes and their substrates is a testament to the wonders of molecular biology. Through highly specific interactions and a diverse set of tools, enzyme complexes unlock the secrets of their substrates, facilitating the transformation of molecules into new forms with remarkable precision and efficiency. As we continue to explore this fascinating world, we gain a deeper appreciation for the intricate machinery of life itself.