5 Insights on Effective Nuclear Charge Trends
Understanding Nuclear Charge and Its Impact on Atoms

Nuclear charge, a fundamental concept in atomic physics, refers to the positive charge within an atom’s nucleus. This charge is pivotal in shaping the behavior and properties of atoms and their electrons. As we delve deeper into this concept, we uncover its intricate role in the atomic world, providing insights that are crucial for both theoretical understanding and practical applications.
The effective nuclear charge, in particular, is a key factor that influences the behavior of electrons in atoms. It represents the net positive charge experienced by an electron in an atom, taking into account the shielding effect of other electrons. This shielding effect, a fascinating phenomenon, occurs when inner-shell electrons block a portion of the nucleus’s positive charge, effectively reducing the attractive force experienced by outer-shell electrons.
Here are five key insights that shed light on the effective nuclear charge trends:
1. The Shielding Effect and Electron Configuration
The shielding effect, a fundamental concept in atomic physics, is a critical factor in determining the effective nuclear charge an electron experiences. As electrons fill the atomic shells, those in the inner shells shield the outer electrons from the full positive charge of the nucleus. This shielding effect is not uniform; it varies depending on the electron configuration and the specific energy levels of the electrons.
For instance, consider the electron configuration of a sodium atom, which is 1s^2 2s^2 2p^6 3s^1. The 3s^1 electron, being in the outermost shell, is more influenced by the shielding effect of the inner-shell electrons. This results in a reduced effective nuclear charge for this electron, affecting its energy level and behavior.
2. Effective Nuclear Charge and Atomic Size
The effective nuclear charge has a direct impact on the size of an atom. Atoms with a higher effective nuclear charge tend to be smaller, as the increased positive charge attracts electrons more strongly, pulling them closer to the nucleus. Conversely, atoms with a lower effective nuclear charge have their electrons less tightly bound, resulting in larger atomic radii.
Take the elements in the second period of the periodic table as an example. As the effective nuclear charge increases across the period from left to right, the atomic radii decrease. This trend is particularly noticeable when comparing elements like lithium (Li) and fluorine (F). Despite having the same number of electrons, fluorine, with its higher effective nuclear charge, is a smaller atom.
3. Effective Nuclear Charge and Electron Affinity
The effective nuclear charge also plays a significant role in determining an atom’s electron affinity, which is the energy change when an electron is added to a neutral atom to form a negative ion. Atoms with a higher effective nuclear charge generally have a higher electron affinity, as the increased positive charge attracts electrons more strongly.
Consider the elements in Group 17 of the periodic table, also known as the halogens. As we move down the group, the effective nuclear charge decreases due to the increased distance between the outermost electron and the nucleus. This decrease in effective nuclear charge results in a lower electron affinity for elements like chlorine (Cl) compared to its heavier counterpart, iodine (I).
4. Effective Nuclear Charge and Ionization Energy
Ionization energy, the energy required to remove an electron from an atom or ion, is another property influenced by the effective nuclear charge. Atoms with a higher effective nuclear charge typically have higher ionization energies, as the increased positive charge holds the electrons more tightly.
Looking at the first ionization energy of elements in Group 1 of the periodic table, we see a clear trend. As the effective nuclear charge increases from left to right across the period, the ionization energy also increases. This is evident when comparing elements like lithium (Li) and sodium (Na), where the higher effective nuclear charge of sodium results in a higher ionization energy.
5. Effective Nuclear Charge and Chemical Reactivity
The effective nuclear charge is a key factor in determining an atom’s chemical reactivity. Atoms with a higher effective nuclear charge tend to be more chemically reactive, as they have a stronger attraction for electrons. This increased reactivity can lead to more vigorous chemical reactions and the formation of stronger chemical bonds.
Consider the reactivity of elements in Group 1 of the periodic table. As we move down the group, the effective nuclear charge decreases, resulting in a decrease in chemical reactivity. This is evident when comparing the reactivity of lithium (Li) and potassium (K). Despite having one more electron and proton, potassium is less reactive than lithium due to the decreased effective nuclear charge.
Conclusion
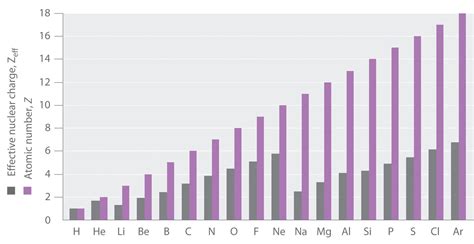
In conclusion, the effective nuclear charge is a crucial concept in atomic physics, influencing various properties and behaviors of atoms. From electron configuration and atomic size to electron affinity and chemical reactivity, the effective nuclear charge plays a central role. Understanding these trends provides valuable insights into the world of atomic physics, offering a deeper understanding of the fundamental building blocks of matter.
As we continue to explore the intricacies of atomic physics, the effective nuclear charge remains a cornerstone concept, guiding our understanding of the atomic world and its fascinating phenomena. With each new discovery, we further unravel the mysteries of the atom, paving the way for advancements in science and technology.



