CS2 Lewis Structure: A Visual Guide
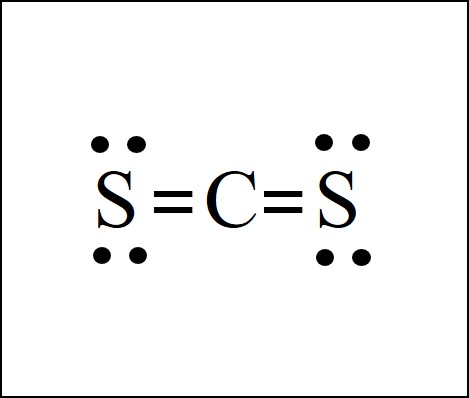
Understanding the CS2 Lewis Structure
The CS2 molecule, also known as carbon disulfide, is an intriguing compound with unique properties and applications. Its Lewis structure provides a visual representation that offers valuable insights into its molecular composition and behavior. In this comprehensive guide, we’ll explore the CS2 Lewis structure, breaking down its complexities and shedding light on its significance in various scientific and industrial domains.
At its core, the CS2 Lewis structure reveals the arrangement of electrons and bonds within the molecule. By analyzing this structure, chemists and researchers can gain a deeper understanding of the molecule’s stability, reactivity, and potential uses. Let’s embark on a journey to unravel the secrets hidden within the CS2 Lewis structure.
The Basics of Lewis Structures
Before delving into the specifics of CS2, let’s briefly review the fundamentals of Lewis structures. Named after Gilbert N. Lewis, who introduced the concept in the early 20th century, Lewis structures are a visual tool used to depict the arrangement of electrons in molecules and ions. These structures use dots or lines to represent electrons and bonds, providing a simple yet powerful way to understand molecular geometry and bonding.
In a Lewis structure, atoms are represented by their chemical symbols, surrounded by dots that signify valence electrons. These dots are arranged in pairs, with single dots representing unpaired electrons and pairs representing shared electrons in covalent bonds. The goal is to ensure that each atom achieves a stable electron configuration, typically following the octet rule, where atoms strive to have eight valence electrons.
Decoding the CS2 Lewis Structure
Now, let’s focus our attention on the CS2 molecule and its unique Lewis structure. CS2, with its carbon-sulfur-carbon framework, presents an intriguing arrangement of electrons and bonds. Here’s how we can decipher its Lewis structure:
Carbon Atoms: Carbon, with its atomic number 6, has four valence electrons. In the CS2 molecule, each carbon atom forms two covalent bonds, resulting in a total of four valence electrons involved in bonding.
Sulfur Atom: Sulfur, with its atomic number 16, has six valence electrons. In CS2, the sulfur atom forms two covalent bonds, contributing two electrons to each bond. This leaves four valence electrons unpaired.
Bonding and Electron Arrangement: In the CS2 Lewis structure, the carbon atoms share their valence electrons with the sulfur atom to form covalent bonds. Each carbon-sulfur bond consists of a double bond, with four shared electrons. The remaining four valence electrons on the sulfur atom are arranged as lone pairs, creating a stable configuration.
Overall Structure: The CS2 molecule exhibits a linear shape, with the carbon atoms positioned at opposite ends and the sulfur atom in the center. This arrangement ensures equal bond lengths and angles, creating a balanced and symmetrical molecule.
The Significance of CS2 Lewis Structure
Understanding the CS2 Lewis structure goes beyond mere visual representation. It provides valuable insights into the molecule’s properties and behavior, which have significant implications in various fields:
Chemical Stability: The Lewis structure reveals the stable electron configuration of CS2, ensuring that each atom has a complete octet. This stability contributes to the molecule’s chemical inertness and resistance to further reactions.
Bonding and Reactivity: The double bonds between carbon and sulfur atoms indicate a strong and stable bond. However, the presence of lone pairs on the sulfur atom suggests potential reactivity with other molecules. CS2 can act as a nucleophile, forming new bonds and participating in various chemical reactions.
Industrial Applications: CS2 is widely used in the production of chemicals, particularly in the manufacturing of viscose rayon, cellophane, and carbon disulfide itself. Understanding its Lewis structure helps chemists optimize reaction conditions and ensure efficient synthesis.
Environmental Impact: CS2 is a volatile organic compound (VOC) and can have adverse environmental effects if not handled properly. Awareness of its Lewis structure aids in developing safe handling and disposal practices, minimizing its impact on air and water quality.
A Historical Perspective
The study of CS2 and its Lewis structure has a rich historical context. Carbon disulfide, first isolated in the early 19th century, has been a subject of fascination for chemists and researchers. Over time, as our understanding of molecular structures evolved, the Lewis structure of CS2 provided a clearer picture of its electron distribution and behavior.
Pioneers like Gilbert N. Lewis and later researchers built upon his work, contributing to our modern understanding of molecular structures. The development of computational tools and advanced spectroscopy techniques further enhanced our ability to analyze and interpret Lewis structures, including that of CS2.
Future Implications and Research
While the CS2 Lewis structure has been well-established, ongoing research continues to explore its intricacies and potential applications. Scientists are investigating the behavior of CS2 in different environments, such as its role in atmospheric chemistry and its interactions with biological systems.
Additionally, the study of CS2 and its derivatives opens up avenues for the development of new materials and technologies. For instance, research into the electronic properties of CS2-based compounds may lead to advancements in organic electronics and energy storage systems.
Conclusion: Unlocking the Secrets of CS2
In conclusion, the CS2 Lewis structure serves as a powerful tool for unraveling the molecular intricacies of carbon disulfide. By visualizing its electron arrangement and bonding, we gain insights into its stability, reactivity, and potential applications. From its role in chemical synthesis to its environmental considerations, understanding CS2’s Lewis structure is a key step in harnessing its power and mitigating its challenges.
As we continue to explore the vast world of molecular structures, the CS2 Lewis structure stands as a testament to the beauty and complexity of chemistry. With each discovery and advancement, we unlock new possibilities and pave the way for a deeper understanding of the universe around us.
The CS2 Lewis structure provides a visual representation of the molecule's electron arrangement, offering insights into its stability, reactivity, and applications in various fields. From chemical synthesis to environmental considerations, understanding CS2's Lewis structure is essential for unlocking its potential.
How does the CS2 Lewis structure contribute to our understanding of the molecule’s behavior?
+The CS2 Lewis structure reveals the molecule’s electron configuration, bonding pattern, and stability. This information helps chemists predict its reactivity, understand its role in chemical reactions, and optimize its applications in various industries.
What are the key features of the CS2 Lewis structure?
+The CS2 Lewis structure showcases the linear arrangement of carbon-sulfur-carbon atoms, with double bonds between carbon and sulfur. It also highlights the presence of lone pairs on the sulfur atom, indicating potential reactivity.
How does the Lewis structure of CS2 compare to other molecules?
+CS2’s Lewis structure differs from simpler molecules like water (H2O) or methane (CH4) due to its double bonds and lone pairs. This complexity gives CS2 unique chemical properties and makes it a versatile compound in various applications.
What are some practical applications of CS2, as revealed by its Lewis structure?
+CS2’s Lewis structure indicates its potential as a nucleophile, making it useful in chemical synthesis. It is also a key intermediate in the production of viscose rayon and cellophane, showcasing its industrial significance.



