3 Common Ion Effect Tips
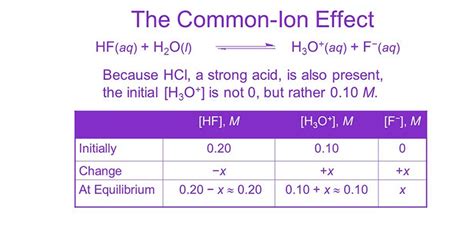
The common ion effect is a fundamental concept in chemistry that influences solubility and reaction dynamics. Here are three essential tips to grasp this phenomenon better:
Understand the Nature of Common Ions: Common ions are ions that are present in a solution and can originate from multiple sources. These ions often come from the dissociation of the solute itself or from an external source, such as an added salt. For instance, when sodium chloride (NaCl) is dissolved in water, it dissociates into sodium ions (Na⁺) and chloride ions (Cl⁻). These ions are now common to the solution.
Impact on Solubility: The presence of a common ion can significantly impact the solubility of a compound. According to Le Chatelier’s principle, adding a common ion to a saturated solution will shift the equilibrium towards the reactants, thereby decreasing the solubility of the compound. This effect is particularly noticeable in the solubility of sparingly soluble salts. For example, if we add sodium chloride to a solution containing lead(II) chloride (PbCl₂), the common chloride ions will decrease the solubility of PbCl₂.
Application in Qualitative Analysis: The common ion effect is a powerful tool in qualitative analysis, especially in the identification of cations and anions. By adding a known common ion to a solution containing an unknown compound, we can observe the changes in solubility or the formation of a precipitate. This method is commonly used in laboratory experiments to identify the presence of specific ions.
The common ion effect is a subtle yet crucial aspect of chemistry. It demonstrates the interconnectedness of ions in a solution and how they influence each other's behavior. Understanding this concept is essential for predicting and controlling reactions, especially in analytical chemistry and industrial processes.
Pros
- Helps control and predict solubility changes.
- Aids in the identification of ions in qualitative analysis.
- Allows for precise manipulation of reaction conditions.
Cons
- Can complicate solubility calculations if not considered.
- May lead to unexpected results if the presence of common ions is overlooked.
How does the common ion effect impact the solubility of a compound?
+The common ion effect decreases the solubility of a compound by shifting the equilibrium towards the reactants. This is because the addition of a common ion decreases the activity of the ions in the solution, thereby reducing the driving force for the compound to dissolve.
<div class="faq-item">
<div class="faq-question">
<h3>Can the common ion effect be used to increase solubility?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>No, the common ion effect inherently decreases solubility. However, this effect can be utilized to selectively precipitate or dissolve specific ions in a mixture by carefully controlling the concentration of common ions.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What are some real-world applications of the common ion effect?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>The common ion effect is crucial in water treatment, where it's used to control the solubility of salts and remove contaminants. It's also employed in the pharmaceutical industry to control the dissolution rate of drugs and in analytical chemistry for ion identification.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>Are there any exceptions to the common ion effect rule?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Yes, there are some exceptions, particularly with complex ions and coordination compounds. In these cases, the common ion effect may not follow the typical rules due to the formation of stable complexes.</p>
</div>
</div>
</div>