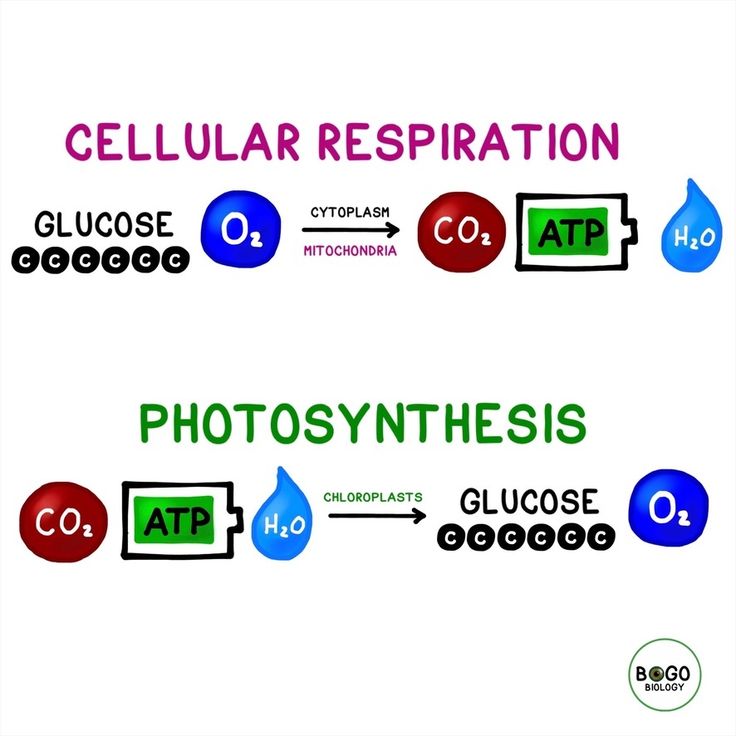
The Chemical Equation of Photosynthesis

Photosynthesis, the remarkable process by which plants harness sunlight to convert carbon dioxide and water into oxygen and energy-rich glucose, is a cornerstone of life on Earth. At its core lies a complex chemical equation, a fundamental representation of this vital biological reaction. Understanding this equation is not merely an academic pursuit; it offers profound insights into the very mechanisms that sustain our planet’s ecosystems and, ultimately, our existence.
This chemical equation is more than just a collection of symbols and reactions; it encapsulates the essence of a process that has shaped the course of life's evolution and continues to sustain it today.
Unraveling the Equation

The chemical equation for photosynthesis is often presented as a straightforward reaction:
\[ \begin{equation*} 6\mathrm{CO_2} + 6\mathrm{H_2O} \overset{\text{Light Energy}}{\longrightarrow} \mathrm{C_6H_{12}O_6} + 6\mathrm{O_2} \,. \end{equation*} \]
Here, six molecules of carbon dioxide ($\mathrm{CO_2}) and six molecules of water (\mathrm{H_2O}) combine in the presence of light energy to produce one molecule of glucose (\mathrm{C_6H_{12}O_6}) and six molecules of oxygen (\mathrm{O_2}$). This seemingly simple equation, however, belies the intricate molecular dance that occurs within plant cells, particularly in specialized organelles called chloroplasts.
The Role of Chloroplasts
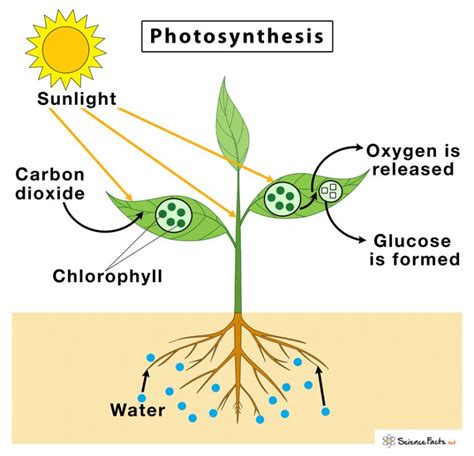
Chloroplasts are the powerhouses of photosynthesis, containing a pigment called chlorophyll, which captures light energy. This energy is then used to drive the complex series of reactions that comprise photosynthesis. The process can be divided into two main stages: the light-dependent reactions and the light-independent reactions, or the Calvin cycle.
Light-Dependent Reactions
In the light-dependent reactions, light energy is absorbed by chlorophyll molecules, exciting electrons to higher energy levels. These energized electrons are then used to generate ATP (adenosine triphosphate) and NADPH (nicotinamide adenine dinucleotide phosphate), two crucial energy carriers. This stage also involves the splitting of water molecules, releasing oxygen as a byproduct and providing hydrogen ions (protons) that contribute to the formation of ATP.
\[ \begin{equation*} \mathrm{H_2O} \overset{\text{Light Energy}}{\longrightarrow} \mathrm{O_2} + 2\mathrm{H^+} + 2\mathrm{e^-} \,. \end{equation*} \]
Calvin Cycle (Light-Independent Reactions)
The Calvin cycle, a series of biochemical reactions, uses the ATP and NADPH generated in the light-dependent reactions to convert carbon dioxide into organic compounds, primarily glucose. This cycle involves three main steps: carbon fixation, reduction, and regeneration.
Carbon Fixation: Carbon dioxide is combined with a five-carbon sugar, RuBP (ribulose-1,5-bisphosphate), to form a six-carbon intermediate. This intermediate is then split into two three-carbon molecules called 3-PGA (3-phosphoglycerate).
Reduction: With the help of ATP and NADPH, the 3-PGA molecules are converted into another three-carbon molecule, G3P (glyceraldehyde-3-phosphate). This molecule is the building block for glucose synthesis.
Regeneration: Some of the G3P molecules are used to regenerate RuBP, allowing the cycle to continue. The remaining G3P molecules are combined to form glucose and other organic compounds.
The Importance of Light and Pigments
Light energy is the catalyst that drives photosynthesis, but not all light is equal. Different pigments, such as chlorophyll a, chlorophyll b, and various carotenoids, are responsible for absorbing specific wavelengths of light. These pigments are arranged in complexes called photosystems, which capture and utilize light energy with remarkable efficiency.
The Diversity of Pigments
The variety of pigments allows plants to harness a broader spectrum of light, increasing their efficiency in different light conditions. For example, carotenoids can absorb longer wavelengths of light, providing an advantage in low-light environments.
Competition for Light
While the diversity of pigments is beneficial, it can also lead to competition within the plant. Different pigments may absorb light more efficiently at different wavelengths, leading to a complex interplay for optimal light utilization.
Factors Affecting Photosynthesis
Several factors influence the rate and efficiency of photosynthesis, including:
Light Intensity and Quality: As mentioned, different pigments absorb different wavelengths of light. Therefore, the intensity and spectrum of light can significantly impact the rate of photosynthesis.
Temperature: Photosynthesis is sensitive to temperature. While an increase in temperature generally accelerates enzyme activity, excessively high temperatures can denature enzymes, slowing or halting the process.
Carbon Dioxide Availability: Adequate levels of carbon dioxide are essential for photosynthesis. In environments with low carbon dioxide concentrations, the rate of photosynthesis may be limited.
Water Availability: Water is a critical reactant in photosynthesis, and its availability can influence the process. Plants have various adaptations, such as deep root systems or water-storage mechanisms, to ensure access to water.
Practical Applications and Future Considerations
Understanding the chemical equation of photosynthesis has far-reaching implications. It provides insights into crop management, enabling farmers to optimize growing conditions for maximum yield. Additionally, it informs efforts in biotechnology and synthetic biology, where scientists aim to enhance photosynthesis in crops or even develop artificial photosynthetic systems.
As we face global challenges like climate change and the need for sustainable energy sources, the study of photosynthesis takes on new urgency. Researchers are exploring ways to harness the efficiency of natural photosynthesis for applications in renewable energy, such as artificial photosynthesis systems that could convert sunlight directly into fuel.
Conclusion
The chemical equation of photosynthesis is a testament to the intricate beauty of nature’s design. It encapsulates a process that has sustained life on Earth for millions of years and continues to be a source of inspiration and innovation in the fields of biology, agriculture, and renewable energy. As we unravel the complexities of this equation, we not only deepen our understanding of the natural world but also uncover potential solutions to some of humanity’s most pressing challenges.



