Unveiling the Lewis Structure of CCl4
The molecular world is an intricate dance of atoms and their interactions, and one such intriguing molecule is CCl4, or carbon tetrachloride. This seemingly simple compound, with its four chlorine atoms surrounding a central carbon, holds a wealth of chemical secrets. Let’s embark on a journey to uncover the Lewis structure of CCl4, a fundamental step in understanding its unique properties and behavior.
The Basics of Lewis Structures

Before we dive into the specifics of CCl4, it’s essential to grasp the concept of Lewis structures, a fundamental tool in chemistry. Named after Gilbert N. Lewis, the American chemist who proposed this idea in 1916, Lewis structures provide a visual representation of the arrangement of atoms in a molecule and the distribution of electrons between them. These structures offer a simplified view of molecular bonding, helping us understand how elements combine to form stable compounds.
In a Lewis structure, atoms are represented by their chemical symbols, and the bonds between them are denoted by lines or dots. Each line represents a shared pair of electrons, also known as a covalent bond. Dots, on the other hand, represent lone pairs of electrons that are not involved in bonding but contribute to the stability of the molecule.
Deconstructing CCl4
Now, let’s turn our attention to the star of our show, CCl4. Carbon tetrachloride is a colorless, dense liquid with a distinctive odor. It was once widely used as a refrigerant, a dry cleaning solvent, and even in fire extinguishers. However, due to its harmful environmental impact and potential health risks, its use has been significantly reduced.
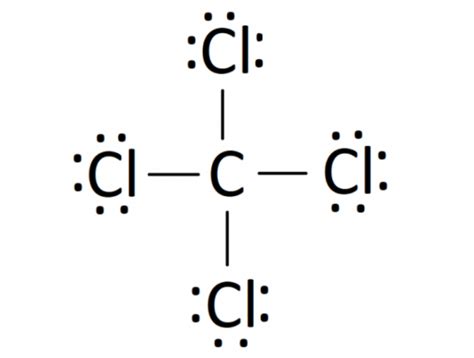
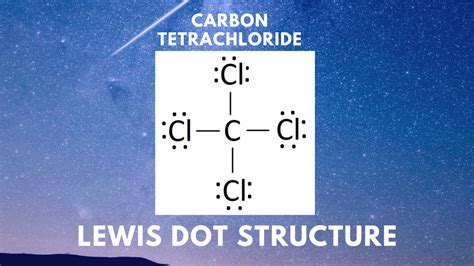
The Lewis structure of CCl4 is a fascinating puzzle to solve. With four chlorine atoms surrounding a central carbon, we must understand how these atoms share electrons to form stable bonds. Carbon, being a group 14 element, typically forms four covalent bonds. Chlorine, on the other hand, being a group 17 element, has a strong affinity for electrons and is keen to acquire an octet.
The Octet Rule in Action
One of the fundamental principles in chemistry, the octet rule, states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration similar to that of a noble gas, which has a complete outer electron shell. In the case of CCl4, both carbon and chlorine aim to fulfill this rule.
Carbon, with its four valence electrons, needs to gain four more electrons to complete its octet. Chlorine, with seven valence electrons, needs to gain one more electron to achieve a stable octet. So, in CCl4, carbon donates one electron to each of the four chlorine atoms, and in return, each chlorine atom donates one electron to carbon.
Visualizing the Lewis Structure
To visualize the Lewis structure of CCl4, we can draw it as follows:
Carbon (C) and chlorine (Cl) atoms are represented by their chemical symbols, with a single line connecting each chlorine atom to the central carbon atom. This line represents a shared pair of electrons, or a covalent bond. Each chlorine atom has three lone pairs of electrons, denoted by dots, while the central carbon atom has no lone pairs.
CCl4 follows the molecular geometry of a tetrahedral shape, with the four chlorine atoms positioned at the corners of a tetrahedron and the carbon atom at the center. This arrangement ensures that each atom's octet is satisfied, and the molecule as a whole is stable.
The Bonding Pattern

In CCl4, each carbon-chlorine bond is a single covalent bond, represented by a single line in the Lewis structure. These bonds are formed by the sharing of electrons between the carbon and chlorine atoms. The carbon atom donates one electron to each chlorine atom, and in return, each chlorine atom donates one electron to carbon.
The Stability of CCl4
The Lewis structure of CCl4 beautifully illustrates how the molecule achieves stability through the octet rule. Each atom, carbon and chlorine, satisfies its need for a complete outer electron shell. This stability makes CCl4 a relatively unreactive compound at room temperature. However, under certain conditions, such as high temperatures or in the presence of catalysts, CCl4 can undergo decomposition, releasing toxic gases like phosgene and hydrogen chloride.
Historical Context and Environmental Impact
CCl4 has an interesting history. Its discovery and widespread use in the early 20th century revolutionized industries like refrigeration and dry cleaning. However, its environmental impact soon became a concern. CCl4 is a persistent organic pollutant, meaning it can remain in the environment for long periods, accumulating in ecosystems. It was found to be a significant contributor to ozone depletion, leading to the ban of its production and use in many countries under the Montreal Protocol.
Future Trends and Applications
Despite its environmental concerns, CCl4 still finds niche applications in certain industries. It is used as a precursor in the synthesis of other compounds, such as chlorinated solvents and pesticides. Additionally, its unique properties, like its density and low flammability, make it valuable in specific laboratory settings.
As we move forward, researchers are exploring alternatives to CCl4, aiming to develop environmentally friendly compounds with similar properties. This pursuit reflects a broader trend in chemistry, where sustainability and green chemistry practices are gaining prominence.
Conclusion
Unveiling the Lewis structure of CCl4 takes us on a journey from the basics of chemical bonding to the intricate dance of electrons within a molecule. This simple yet powerful tool, the Lewis structure, allows us to understand the stability and behavior of molecules like CCl4, contributing to our broader understanding of the molecular world. As we continue to explore and innovate, the principles we uncover through such analyses will guide us toward a more sustainable and responsible future.



