How to Calculate Partial Pressure
Understanding the concept of partial pressure is crucial in various scientific fields, especially when dealing with gas mixtures. It’s a fundamental principle that allows us to analyze and predict the behavior of gases in different environments. So, let’s dive into the process of calculating partial pressure and explore its significance.
What is Partial Pressure?

Partial pressure refers to the pressure exerted by an individual gas within a mixture of gases. Imagine a balloon filled with a mixture of helium and oxygen. The partial pressure of helium represents the pressure that helium alone would exert if it occupied the entire volume of the balloon. It’s like isolating one gas and considering its impact on the total pressure.
Partial pressure is a key concept in gas behavior and is essential for understanding how gases interact in various applications, from medical procedures to industrial processes.
The Dalton’s Law of Partial Pressures

Dalton’s Law, proposed by John Dalton in the early 19th century, provides the foundation for calculating partial pressures. According to this law, the total pressure of a gas mixture is the sum of the partial pressures of its individual components. In mathematical terms:
\[ \begin{equation*} P_{\text{total}} = P_1 + P_2 + P_3 + ... + P_n \end{equation*} \]
Where: - P_{\text{total}} is the total pressure of the gas mixture. - P_1, P_2, P_3, ... P_n are the partial pressures of each individual gas in the mixture.
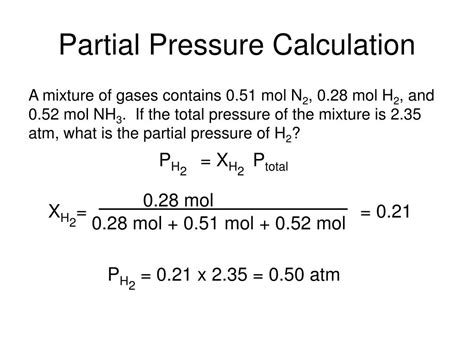
Calculating Partial Pressure
To calculate the partial pressure of a specific gas in a mixture, you need to know two key pieces of information: 1. Mole Fraction: The mole fraction (x) of a gas represents the number of moles of that gas divided by the total number of moles in the mixture. Mathematically: $ \begin{equation*} x_i = \frac{\text{moles of gas } i}{\text{total moles in the mixture}} \end{equation*} 2. Total Pressure: The total pressure (P_{\text{total}}$) of the gas mixture, which can be measured or provided in the problem statement.
With these values, you can calculate the partial pressure (P_i) of any gas in the mixture using the following formula:
\[ \begin{equation*} P_i = x_i \cdot P_{\text{total}} \end{equation*} \]
Example Scenario:
Let’s say we have a gas mixture in a closed container at a total pressure of 1000 mmHg. The mixture consists of oxygen (O_2) and nitrogen (N_2), with mole fractions of 0.2 for oxygen and 0.8 for nitrogen.
Step 1: Identify the mole fractions and total pressure: - Mole fraction of oxygen (x_{O_2}) = 0.2 - Mole fraction of nitrogen (x_{N_2}) = 0.8 - Total pressure (P_{\text{total}}) = 1000 mmHg
Step 2: Calculate the partial pressures: - Partial pressure of oxygen (P_{O_2}): $ \begin{align*} P_{O_2} &= x_{O_2} \cdot P_{\text{total}} \\ &= 0.2 \cdot 1000 \text{ mmHg} \\ &= 200 \text{ mmHg} \end{align*} $
- Partial pressure of nitrogen (P_{N_2}): $ \begin{align*} P_{N_2} &= x_{N_2} \cdot P_{\text{total}} \\ &= 0.8 \cdot 1000 \text{ mmHg} \\ &= 800 \text{ mmHg} \end{align*} $
Real-World Applications:

- Medical: In medical settings, understanding partial pressures is crucial for administering gases like oxygen and nitrous oxide safely.
- Industrial Processes: Partial pressure calculations are essential in chemical engineering, especially when dealing with gas separation and purification processes.
- Environmental Science: Scientists use partial pressure concepts to study atmospheric chemistry and the behavior of greenhouse gases.
Key Takeaway:
Partial pressure calculations provide a quantitative way to understand the behavior of individual gases within a mixture. This knowledge is fundamental in various scientific and industrial applications, ensuring safe and efficient operations.
How does partial pressure affect gas solubility in liquids?
+Partial pressure plays a significant role in gas solubility. According to Henry’s Law, the amount of gas dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid. This principle is crucial in understanding how gases like oxygen and carbon dioxide dissolve in water, impacting aquatic ecosystems and human physiology.
Can partial pressure calculations be applied to real-time gas monitoring systems?
+Absolutely! Real-time gas monitoring systems often rely on partial pressure calculations to provide accurate readings. These systems are used in industries like manufacturing and healthcare to ensure safety and maintain optimal gas conditions.
What are some common units used for measuring partial pressure?
+Common units for partial pressure include millimeters of mercury (mmHg), pascals (Pa), atmospheres (atm), and kilopascals (kPa). The choice of unit depends on the context and industry standards.
How does temperature affect partial pressure calculations?
+Temperature can influence gas behavior and, consequently, partial pressure calculations. The ideal gas law, which relates pressure, volume, temperature, and moles, plays a crucial role in understanding how temperature changes impact gas mixtures.



