Discovering pH at the Equivalence Point

Welcome to a comprehensive exploration of the crucial concept in acid-base titration: discovering pH at the equivalence point. This journey delves into the heart of analytical chemistry, offering a deep understanding of this fundamental aspect of chemical reactions. We'll navigate through the intricacies of pH measurements, the significance of the equivalence point, and the various methods employed to determine it, all while uncovering the practical applications and real-world implications.
Understanding the Equivalence Point

The equivalence point in an acid-base titration marks a pivotal moment when the number of moles of acid being titrated equals the number of moles of the titrant, typically a strong base. This event signifies the complete neutralization of the acid, resulting in a solution that is neither acidic nor basic, often referred to as a buffer solution. This point is not merely a theoretical concept but a practical milestone with significant implications in chemistry and various industrial processes.
The Importance of Precision
Accurate determination of the equivalence point is crucial for several reasons. Firstly, it provides a precise endpoint for the titration process, ensuring reliable results. Secondly, it enables the calculation of the acid or base’s concentration, a fundamental parameter in chemistry. Lastly, in industrial settings, this precision is vital for quality control, ensuring products meet specific pH requirements.
Methods to Determine pH at Equivalence

Several techniques are employed to pinpoint the pH at the equivalence point, each offering unique advantages and considerations. Let’s explore these methods in detail.
Indicator-Based Methods
Perhaps the most common and accessible approach is the use of pH indicators. These indicators, such as phenolphthalein or methyl orange, undergo a color change at specific pH ranges. By adding an indicator to the titration solution and observing the color change, one can estimate the pH at the equivalence point. This method, while simple, relies on the indicator’s accuracy and may not provide precise results for complex reactions.
pH Meters and Electrodes
For more precise measurements, pH meters equipped with glass electrodes are employed. These devices measure the potential difference between the glass electrode and a reference electrode, which is then converted to pH. This method offers high accuracy and is particularly useful for continuous monitoring during titrations. However, pH meters require regular calibration and may be more expensive than indicator-based methods.
| Indicator | Color Change Range |
|---|---|
| Phenolphthalein | Colorless to Pink (8.2 - 10.0) |
| Methyl Orange | Red to Yellow (3.1 - 4.4) |

Conductivity Measurements
In certain cases, the equivalence point can be determined by measuring the electrical conductivity of the solution. As the titration progresses, the conductivity changes due to the formation of ionic species. This method is particularly useful for strong acid-strong base titrations. However, it requires specialized equipment and may not be suitable for all titration types.
Potentiometric Titration
Potentiometric titration involves measuring the potential difference between two electrodes immersed in the solution. This method provides continuous data, allowing for the precise determination of the equivalence point. It is highly accurate and can be used for a wide range of titration types. However, the setup and interpretation of results require specialized knowledge.
Real-World Applications
The ability to accurately determine pH at the equivalence point has profound implications across various industries and scientific fields.
Pharmaceuticals and Healthcare
In the pharmaceutical industry, the pH of a drug formulation can significantly impact its efficacy and stability. Accurate pH measurements at the equivalence point ensure the drug’s quality and performance. Similarly, in healthcare, maintaining the correct pH is crucial for diagnostic tests and therapeutic procedures.
Environmental Science
Environmental scientists use pH measurements to assess water quality and soil health. The equivalence point determination helps in understanding the acidity or alkalinity of natural systems, which is vital for ecological balance and pollution control.
Food and Beverage Industry
The food and beverage sector relies on precise pH control for product quality and safety. From ensuring the right acidity in beverages to controlling fermentation processes, accurate pH measurements are essential.
Chemical Manufacturing
In chemical manufacturing, the equivalence point is crucial for quality control and process optimization. It ensures that chemical reactions proceed as intended, maintaining product consistency and safety.
Future Implications and Innovations
As technology advances, the field of pH measurement and titration continues to evolve. New techniques and instruments are being developed to enhance precision and efficiency.
Smart pH Sensors
The integration of IoT (Internet of Things) technology into pH sensors is revolutionizing the field. These smart sensors can transmit real-time pH data, allowing for remote monitoring and control. This technology is particularly beneficial for industrial processes and environmental monitoring.
Microfluidic Devices
Microfluidic devices, which handle tiny volumes of fluids, are being explored for their potential in titration and pH measurement. These devices offer high precision and are suitable for point-of-care testing and lab-on-a-chip applications.
Artificial Intelligence
AI is being employed to interpret titration data and predict equivalence points. Machine learning algorithms can analyze complex reactions and provide accurate pH estimates, offering a new dimension to titration analysis.
Conclusion
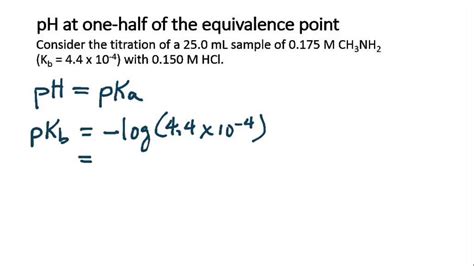
The journey to discover pH at the equivalence point is a testament to the complexity and elegance of chemistry. From simple indicator methods to advanced AI-driven techniques, the evolution of pH measurement reflects the progress and innovation in the field. As we continue to explore and innovate, the understanding and control of pH will undoubtedly play a pivotal role in shaping the future of science and industry.
What is the difference between a strong acid and a weak acid in titration?
+Strong acids, such as hydrochloric acid, completely dissociate into ions in water, while weak acids, like acetic acid, only partially dissociate. This difference affects the pH and behavior of the solution during titration.
How does the choice of indicator affect the accuracy of pH measurement at the equivalence point?
+The choice of indicator is critical. The indicator should have a color change range that corresponds to the expected pH at the equivalence point. If the indicator’s range does not align, it may lead to inaccurate results.
What are some common applications of pH titration in everyday life?
+pH titration is used in various ways in daily life, from testing the acidity of fruit juices and soft drinks to monitoring the pH of swimming pools and aquariums. It’s also crucial in home brewing and winemaking for quality control.