Br Electron Configuration Explained Simply

Understanding the Basics of Electron Configuration
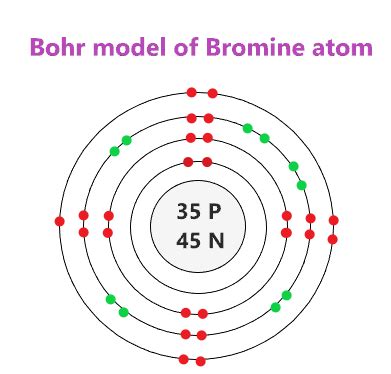
At the heart of every atom lies a complex dance of electrons, orchestrating the very fabric of matter. The electron configuration, a fundamental concept in chemistry, provides us with a blueprint of how these electrons are arranged, offering profound insights into the behavior and properties of elements.
In this article, we’ll embark on a journey to unravel the mysteries of electron configuration, demystifying its principles and shedding light on its profound implications in the realm of atomic and molecular science.
The Electron Configuration Concept
Electron configuration is a descriptive way of arranging the electrons of an atom, depicting how these negatively charged particles are distributed across various energy levels and orbitals. Think of it as a cosmic map, guiding us through the intricate pathways electrons take within an atom’s nucleus.
The concept of electron configuration is akin to understanding the seating arrangement in a theater. Each energy level represents a different section, while orbitals within each level signify specific seats. Electrons, the audience in this analogy, fill these seats in a particular order, dictated by energy and stability considerations.
Historical Evolution of Electron Configuration Theory
The theory of electron configuration has its roots in the late 19th and early 20th centuries, evolving from groundbreaking discoveries in atomic physics. Scientists like J.J. Thomson, Neils Bohr, and Erwin Schrödinger laid the foundation for our understanding of electron behavior.
Bohr’s model, a pivotal advancement, proposed that electrons occupy distinct energy levels or shells around the nucleus, each with a specific capacity. This model, though simplified, marked a significant leap forward in explaining atomic structure.
The Aufbau Principle: Building Electron Configurations
The Aufbau principle, a cornerstone of electron configuration theory, dictates the order in which electrons fill atomic orbitals. It’s a systematic approach, much like constructing a building brick by brick.
Pros of the Aufbau Principle
- Provides a logical, step-by-step process for electron arrangement.
- Facilitates easy prediction of electron configurations for most elements.
- Offers insights into the stability and reactivity of atoms.
Cons of the Aufbau Principle
- May not accurately predict configurations for certain transition metals.
- Doesn't account for the complexity of electron behavior in highly charged ions.
- Simplifies the dynamic nature of electron arrangements in complex systems.
Electron Configuration Notation: Unraveling the Code
Electron configuration is typically expressed using a specialized notation, a concise language that communicates the arrangement of electrons. Here’s a breakdown of this notation:
- Energy Levels: Represented by the principal quantum number (n), energy levels are designated by whole numbers (1, 2, 3, etc.).
- Orbitals: Within each energy level, there are sub-levels or orbitals, denoted by letters (s, p, d, f). Each orbital has a specific shape and energy.
- Electrons: Electrons are represented by superscripts, indicating the number of electrons in a particular orbital.
For instance, the electron configuration of carbon © is 1s² 2s² 2p². This notation signifies that the first energy level (n=1) is filled with two electrons (s²), while the second level (n=2) has two electrons in the s orbital and two in the p orbital.
Comparative Analysis: Electron Configuration in Different Elements
Let’s explore how electron configuration varies across different elements, offering a glimpse into the unique electronic structures that underpin the diversity of the periodic table:
| Element | Atomic Number | Electron Configuration |
|---|---|---|
| Hydrogen (H) | 1 | 1s¹ |
| Helium (He) | 2 | 1s² |
| Lithium (Li) | 3 | 1s² 2s¹ |
| Carbon (C) | 6 | 1s² 2s² 2p² |
| Oxygen (O) | 8 | 1s² 2s² 2p⁴ |
| Neon (Ne) | 10 | 1s² 2s² 2p⁶ |
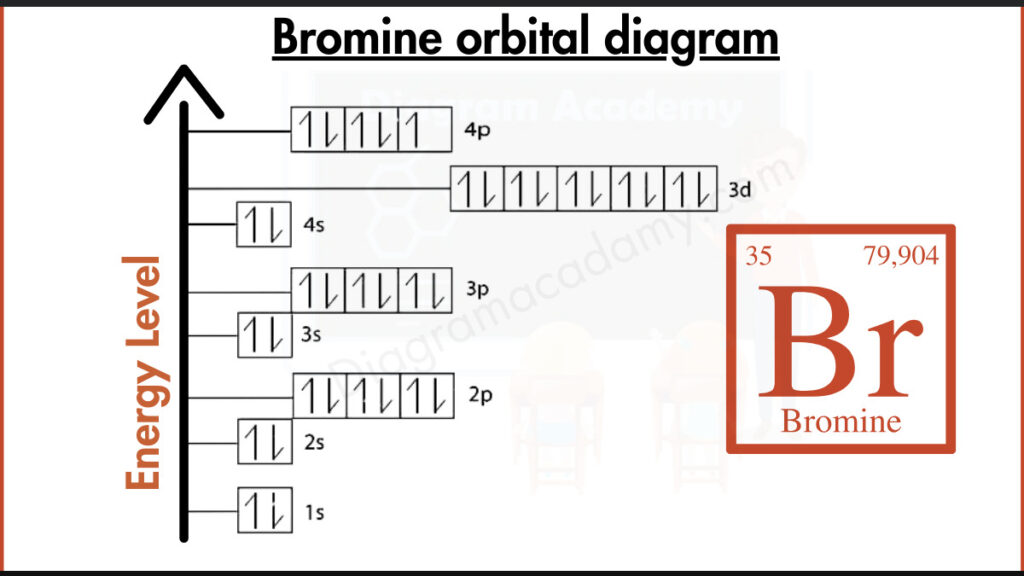
The Impact of Electron Configuration on Atomic Properties
Electron configuration is not merely an abstract concept but has tangible implications for the properties of atoms and molecules. It influences:
- Chemical Reactivity: Elements with similar electron configurations often exhibit similar chemical behaviors, forming predictable bonds and reactions.
- Atomic Radius: The distribution of electrons across energy levels affects the size of atoms, with larger energy levels accommodating more electrons and resulting in larger atomic radii.
- Ionization Energy: The energy required to remove an electron from an atom is influenced by its electron configuration, particularly the stability of the outermost orbital.
Future Trends and Research Directions
While electron configuration provides a robust framework for understanding atomic structure, ongoing research continues to refine and expand our knowledge. Future trends include:
- Quantum Mechanics: Advancements in quantum mechanics offer deeper insights into the probabilistic nature of electron behavior, providing a more nuanced understanding of electron configuration.
- Computational Chemistry: The use of advanced computational techniques enables the simulation and prediction of electron configurations for complex molecules, facilitating the design of novel materials and compounds.
Conclusion

In conclusion, electron configuration serves as a powerful tool for deciphering the intricate world of atomic and molecular science. By understanding how electrons are arranged within atoms, we gain profound insights into the properties and behaviors of elements, paving the way for innovative advancements in chemistry, materials science, and beyond.
The journey into the heart of electron configuration is a testament to the human spirit of exploration and our relentless pursuit of understanding the fundamental building blocks of our universe.



