5 Factors Affecting Water's Freezing Point
Water, a seemingly simple substance, has complex properties, and its freezing point is influenced by various factors. Understanding these influences is crucial, especially in fields like environmental science, agriculture, and even everyday life. Here, we delve into five key factors that determine when water transitions from a liquid to a solid state.
1. Temperature: The Primary Determinant
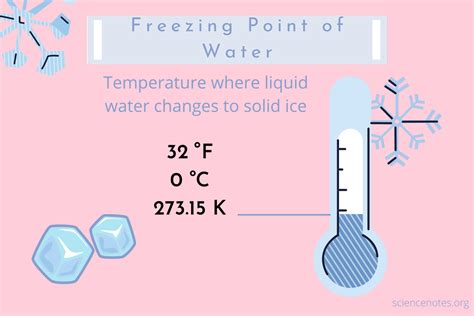
Undoubtedly, temperature takes center stage when discussing water’s freezing point. This fundamental concept is rooted in the molecular behavior of water. As temperatures drop, the kinetic energy of water molecules decreases, leading to slower movement and, eventually, the formation of a crystalline structure—ice.
For instance, consider a body of water like a lake. During winter, as the air temperature consistently falls below freezing (0°C or 32°F), the water’s surface begins to solidify, creating a layer of ice. This process occurs gradually, starting from the top and moving downward.
The freezing point of water is a critical factor in various natural phenomena. For instance, the formation of ice caps and glaciers is influenced by extended periods of sub-zero temperatures. Conversely, understanding how water freezes can also help predict and mitigate the impact of extreme weather events, like flash floods, which can occur when ice melts rapidly.
2. Pressure: The Hidden Influencer
While temperature is the primary factor, pressure also plays a significant role. At higher pressures, water’s freezing point increases, meaning it requires a lower temperature to freeze. This phenomenon is particularly evident at the bottom of deep bodies of water, such as oceans.
Imagine a scenario where a deep-sea submarine descends to extreme depths. Despite the freezing temperatures at the surface, the water at these depths remains liquid due to the immense pressure. This is because the pressure prevents the water molecules from forming the rigid structure of ice.
Pressure's impact on water's freezing point is a fascinating aspect of physics. It demonstrates how environmental conditions can alter the behavior of substances, offering insights into the complex interactions that shape our world.
3. Impurities: The Disruptive Element
The presence of impurities, such as salts, minerals, or other dissolved substances, can significantly affect water’s freezing point. These impurities disrupt the molecular arrangement of water, hindering the formation of the crystal lattice required for freezing.
Take, for example, the case of seawater. Due to its high salt content, seawater has a lower freezing point compared to pure water. This property is crucial for marine life, as it allows seawater to remain liquid at lower temperatures, providing a more stable environment for aquatic organisms.
4. Surface Area: The Role of Exposure
The surface area of water also influences its freezing point. When water is exposed to a larger surface area, it freezes more rapidly. This occurs because a larger surface area provides more sites for ice crystals to form, accelerating the freezing process.
Consider the difference in freezing time between a large lake and a small pond. The lake, with its vast surface area, takes longer to freeze compared to the smaller pond. This principle is utilized in industrial settings, where the design of cooling systems often considers the surface area of water to enhance efficiency.
5. Nucleation Sites: The Catalyst for Freezing
Nucleation sites, such as dust particles or impurities, act as catalysts for the freezing process. These sites provide a surface for water molecules to align and form the crystal lattice structure of ice. The presence of nucleation sites can significantly impact the speed and uniformity of freezing.
Imagine a scenario where a cloud, containing numerous tiny water droplets, encounters freezing temperatures. The presence of dust particles within the cloud acts as nucleation sites, causing the water droplets to freeze and form ice crystals. This process is fundamental to the formation of snow and other forms of precipitation.
How does temperature affect water’s freezing point?
+Temperature is the primary factor determining water’s freezing point. As temperatures drop, the kinetic energy of water molecules decreases, leading to slower movement and the formation of ice. This process occurs gradually, starting from the surface and moving downward.
Can water freeze at higher pressures?
+Yes, at higher pressures, water’s freezing point increases. This means that water can remain liquid at temperatures below freezing due to the pressure preventing the formation of the ice crystal lattice.
How do impurities affect water’s freezing point?
+Impurities, like salts or minerals, disrupt the molecular arrangement of water, hindering the formation of the crystal lattice required for freezing. This results in a lower freezing point, as seen in the case of seawater.
Does surface area influence water’s freezing speed?
+Absolutely! A larger surface area provides more sites for ice crystals to form, accelerating the freezing process. This principle is utilized in industrial cooling systems to enhance efficiency.
What are nucleation sites, and how do they impact freezing?
+Nucleation sites, such as dust particles or impurities, act as catalysts for freezing. They provide a surface for water molecules to align and form the crystal lattice structure of ice, influencing the speed and uniformity of freezing.